Genevieve Bates

Project : Molecular mechanics of myosin in muscular dystrophy
I studied muscular dystrophy at the molecular level using the mdx (dystrophic) mouse model. Since, it is believed that the lack of the protein dystrophin in Duchenne muscular dystrophy may affect muscle proteins such as the myosin molecular motor, I used the in vitro motility assay to study disorder at the molecular level. I investigated potential differences in the velocity and force generating capabilities of the protein myosin from healthy and dystrophic mouse diaphragms. Relative force of the two myosins was determined using either the mixed-myosin or the alpha-actinin assay.
As a side project, I investigated the effects of the smooth muscle protein telokin on the mechanics of thiophosphorylated (permanently activated) myosin.
Visual
1)
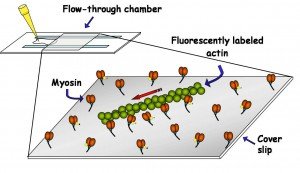
2) Alpha-Actinin Assay: In this assay, alpha-actinin (red anchors) is bound to the coverslip as well as the actin filaments in order to exert a load on the filaments. More alpha-actinin will be required to fully inhibit motility in the presence of a stronger myosin.
3) In Vitro Motility Assay: This assay consists of myosin molecules, randomly adhered to a microscope cover slip, propelling fluorescently labeled actin filaments.
4) Mixed-Myosin Assay: Two different myosins, cycling at different rates, pull on the same actin filament and cause a reduction in velocity proportional to the relative force generated by each type of myosin.
5) *Warshaw figure*
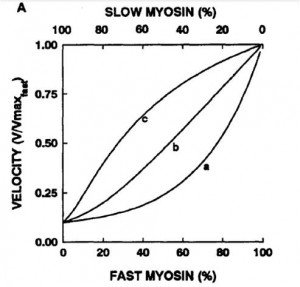
Concentration vs. velocity curve normalized to max
a) slow myosin generates more force
b) both generate same force
c) fast myosin generates more force